A Hot Incandescent Gas Under Low Pressure Produces a Continuous Spectrum
| | Astronomy 161: An Introduction to Solar System Astronomy Prof. Richard Pogge, MTWThF 2:30 |
Lecture 24: Matter & Light
Key Ideas:
Temperature (Kelvin Scale)- Measures internal energy content.
- A hot, dense object produces a continuous spectrum(blackbody spectrum).
- A hot, low-density gas produces an emission-line spectrum.
- A cool, dense gas produces an absorption-line spectrum.
The Interaction of Light & Matter
Light & Matter can interact in a number of different ways:- Matter can transmit light (glass, water).
- Matter can reflect light.
- Matter can gain energy by absorbing light.
- Matter can lose energy by emitting light.
Temperature
Temperature is a measurement of the internal energy content of an object.- Solids:
- Higher temperature means higher average vibrational energy per atom or molecule.
- Gases:
- Higher temperature means more average kinetic energy (faster speeds) per atom or molecule.
Absolute Temperature
- At high temperatures:
- Atoms & molecules move very rapidly.
- At cooler temperatures:
- Atoms & molecules move more slowly.
- Corresponds to a temperature of -273° Celsius (-459° F).
- Called "Absolute Zero".
Kelvin Temperature Scale
An absolute temperature system in which the temperature is directly proportional to the internal energy.- Developed by British physicist William Thomson, First Lord Kelvin (19th century)
- Uses the Celsius degree, but a different zero temperature
Kelvin Absolute Temperature Scale (K):
- 0 K = Absolute Zero
- 273 K = pure water freezes (0° Celsius)
- 373 K = pure water boils (100° C)
We will primarily use the Kelvin scale in this course (and Astronomy 162), but will occasionally use Celsius (with Fahrenheit equivalents) where we are talking about planetary temperatures.
What is a Spectrum?
A spectrum is the distribution of photon energies coming from a light source:- How many photons of each energy are emitted by the light source?
Spectra are observed by passing light through a spectrograph:
- Breaks the light into its component wavelengths and spreads them apart (dispersion).
- Uses either prisms or diffraction gratings.
Kirchoff's Laws of Spectroscopy
- A hot solid or hot, dense gas produces a continuous spectrum.
- A hot, low-density gas produces an emission-line spectrum.
- A continuous spectrum source viewed through a cool, low-density gas produces an absorption-line spectrum.
German physicist Gustav Kirchoff (1824-1887) formulated these laws empirically during the mid-19th century. While they adequately describe the different kinds of spectra that are observed, they do not explain why these spectra appear in these circumstances. A physical explanation had to wait until the 20th century for the development of quantum mechanics and modern atomic theory.
Black Body Radiation
A Blackbody is an object that absorbs all light.- Absorbs at all wavelengths.
As it absorbs light, it heats up.
- Characterized by its Temperature .
It is also the perfect radiator:
- Emits at all wavelengths (continuous spectrum)
- Energy emitted depends strongly on the Temperature.
- Peak wavelength also depends on Temperature.
-
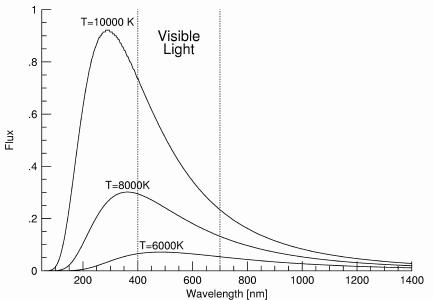
- Blackbody Spectra for three Temperatures: 10000K, 8000K, and 6000K.
Stefan-Boltzmann Law
Energy emitted per second per area by a blackbody with Temperature (T):In Words:
- "Hotter objects are Brighter at All Wavelengths"
Wien's Law
Relates peak wavelength and Temperature:- "Hotter objects are BLUER"
- "Cooler objects are REDDER"
Examples
Person: Body Temperature = 310 K- Peak wavelength = 9400 nm (infrared)
- Typical adults emit about 100 Watts of infrared light.
[Infrared-light image of a familiar person]
Sun: surface temperature = 5770 K
- Peak wavelength = 503 nm (visible light)
- Emits about 3.8x1026 Watts of mostly visible light plus infrared and ultraviolet.
[Visible-light image of a familiar star]
Emission-Line Spectrum
A hot, low-density gas, in which the atoms are relatively isolated from each other, will emit an emission-line spectrum: 
- Only emits light at particular wavelengths, giving the appearance of bright, discrete emission lines.
- There is no light emitted between the emission lines.
19th chemists century noticed that each element, heated into an incandescent gas in a flame, emitted unique emission lines.
- Mapped out the emission-line spectra of known atoms and molecules.
- Used this as a tool to identify the composition of unknown compounds.
- They did not, however, understand how it worked.
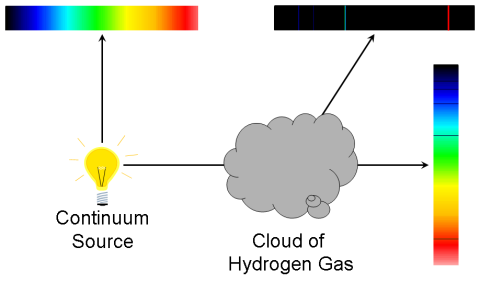
Absorption-Line Spectrum
Light from a continuous spectrum through a vessel containing a cooler gas shows a continuous spectrum from the lamp crossed by of dark absorption lines at particular wavelengths.: 
- The wavelengths of the absorption lines correspond exactly to the wavelengths of emission lines seen when the gas is hot!
- Light is being absorbed by the atoms in the gas.
Why does it work?
Why does each element have a characteristic line spectrum?Answer:
- It is a reflection of the detailed structure of the atom.
- Depends on the number and arrangement of electrons in orbit around the nucleus.
Discovering the reason unlocked the secret of the atom.
Return to [ Unit 4 Index | Astronomy 161 Main Page ]
Updated: 2007 October 19
Copyright � Richard W. Pogge, All Rights Reserved.
Source: https://www.astronomy.ohio-state.edu/pogge.1/Ast161/Unit4/spectra.html

0 Response to "A Hot Incandescent Gas Under Low Pressure Produces a Continuous Spectrum"
Post a Comment